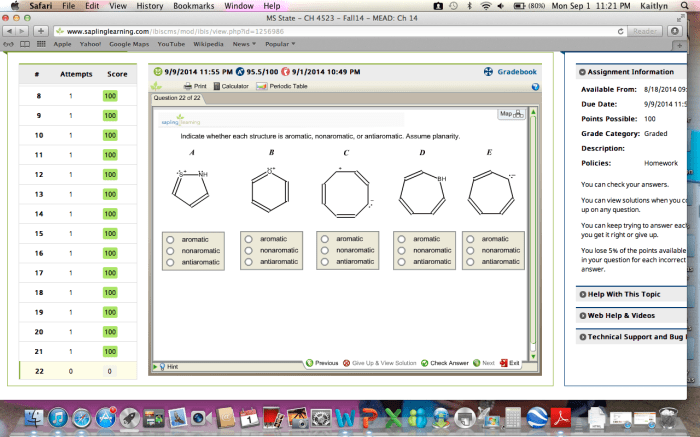
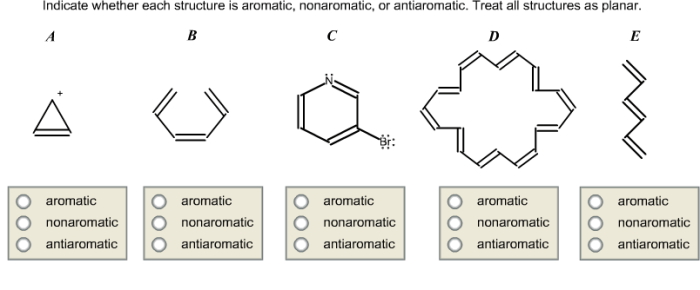
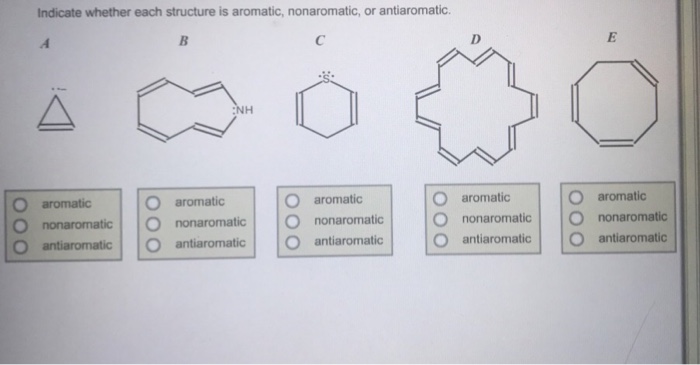
Indicate whether each structure is aromatic nonaromatic or antiaromatic – In the realm of chemistry, the concept of aromaticity plays a pivotal role in understanding the behavior and properties of certain compounds. Delving into the intricacies of aromaticity, this exploration will elucidate the fundamental principles that govern whether a structure exhibits aromatic, nonaromatic, or antiaromatic characteristics, providing a comprehensive overview of this captivating topic.
The journey begins with defining aromaticity, exploring the significance of Hückel’s rule, and delving into the molecular orbital theory. Resonance and delocalization will be examined, followed by an in-depth analysis of antiaromaticity. The prevalence of aromaticity in organic and inorganic compounds will be highlighted, showcasing its diverse applications in various scientific disciplines.
Definition of Aromaticity
Aromaticity is a fundamental concept in chemistry that describes the unique properties and behavior of certain cyclic compounds. Aromatic compounds are characterized by their stability, resonance, and planarity. They exhibit enhanced reactivity and undergo characteristic reactions distinct from their aliphatic counterparts.
Hückel’s Rule
Hückel’s rule is a simple but powerful tool for determining the aromaticity of a cyclic compound. It states that a cyclic compound with (4n + 2) π electrons, where n is an integer, will be aromatic. This rule provides a quick and reliable way to predict the aromatic character of a compound based on its molecular structure.
Molecular Orbital Theory
Molecular orbital theory provides a more detailed understanding of aromaticity. According to this theory, the π electrons in an aromatic compound are delocalized over the entire ring, forming a continuous cloud of electron density. This delocalization results in increased stability and unique chemical properties.
Resonance and Delocalization
Resonance is a key concept in understanding aromaticity. Aromatic compounds often have multiple resonance structures, which contribute to the delocalization of the π electrons. This delocalization stabilizes the compound and enhances its aromatic character.
Antiaromaticity
Antiaromaticity is the opposite of aromaticity. Antiaromatic compounds are cyclic compounds with (4n) π electrons, where n is an integer. These compounds are highly unstable and reactive due to the lack of resonance and delocalization of their π electrons.
Aromaticity in Organic Compounds
Aromaticity is a prevalent feature in organic chemistry. Many organic compounds, such as benzene, naphthalene, and anthracene, exhibit aromatic character. These compounds are found in a wide range of natural products and play essential roles in biological processes.
Aromaticity in Inorganic Compounds
Aromaticity is not limited to organic compounds. Inorganic compounds can also exhibit aromatic character. Examples include borazine, cyclophosphazene, and metallocenes. These inorganic aromatic compounds have unique properties and applications in various fields.
Applications of Aromaticity
Aromaticity has numerous practical applications in various fields. Aromatic compounds are used as solvents, pharmaceuticals, dyes, and materials in industries such as chemistry, pharmaceuticals, and materials science. Their unique properties make them valuable building blocks for the synthesis of complex molecules and functional materials.
General Inquiries: Indicate Whether Each Structure Is Aromatic Nonaromatic Or Antiaromatic
What is the significance of aromaticity in chemistry?
Aromaticity plays a crucial role in determining the stability, reactivity, and properties of certain compounds, influencing their behavior in chemical reactions and their applications in various fields.
How does Hückel’s rule contribute to understanding aromaticity?
Hückel’s rule provides a set of criteria that can be used to predict whether a compound will exhibit aromatic character based on the number of π electrons and the arrangement of double bonds in its structure.
What is the relationship between resonance and aromaticity?
Resonance contributes to aromaticity by delocalizing the π electrons over multiple atoms, resulting in increased stability and enhanced aromatic character.
What are the key characteristics of antiaromatic compounds?
Antiaromatic compounds possess properties that are opposite to those of aromatic compounds, exhibiting higher energy and instability due to the presence of 4n π electrons in a cyclic system.
How is aromaticity utilized in practical applications?
Aromaticity finds applications in various fields, including pharmaceuticals, materials science, and organic synthesis, where aromatic compounds are employed for their unique properties and stability.